Only 6 months left for the transition of all Clinical Trials to CTIS

The deadline for transitioning clinical trials to CTIS ends on January 30, 2025. Studies continuing beyond this date must comply with the Clinical Trials Regulation (CTR), replacing the old CTD. At Sermes CRO, we join the EMA’s effort to communicate this imminent change to clinical trial sponsors in Europe
Celebrating 25 years of innovation in biotechnology: Sermes CRO at the presentation of the Asebio 2023 report

We attended the presentation of the Asebio 2023 Report, an essential event for the Spanish biotechnology sector. This year is particularly special as Asebio celebrates its 25th anniversary, and at Sermes CRO, we are proud to be part of this ecosystem, having been in the industry for 27 years. The advancements and achievements in this sector are impressive, with collaboration being a crucial pillar
26 days to evaluate your clinical trial thanks to AEMPS’ fast-track assessment

The Spanish Agency for Medicines and Health Products (AEMPS) has implemented a new accelerated assessment procedure for certain clinical trials. This initiative aims to make Spain a more attractive environment for innovative drug research. Below, we explain which trials can take advantage of this fast-track process that reduces the evaluation time from 45 to 26 days
The Essential Role of CROs in Clinical Research

From document management to regulatory strategy, CROs are indispensable partners for the advancement of science and medicine. On Clinical Trials Day, join us on this journey to better understand the vital role that CROs play in the development of innovative treatments and medications.
European Artificial Intelligence Regulation: Missed Opportunity or Protective Shield

One sector significantly impacted by the use and development of Artificial Intelligence is healthcare and by extension Clinical Research The new Regulation is crucial for AI development as it affects not only technological advancement but also our fundamental rights
Sermes CRO has become the first Contract Research Organization to embrace Farmaindustria’s Code of Conduct for Personal Data Treatment

The company aligns itself with Farmaindustria’s guidelines, which oversee the handling of personal data in the realms of clinical trials and other clinical and pharmacovigilance research
ICH E9 and CDISC: The Master Formula for Excellence in Clinical Research and Data Management

Clinical studies play a key role in the development of new medicinal products and therapies, as well as in evaluating the efficacy and safety of existing medical treatments. To ensure the quality and reliability of the data collected in these studies, it is essential to follow robust guidelines and standards. One of the most important guidelines in this field is ICH E9, published by the International Council on Harmonization (ICH).
Inspiring diversity, transformative inclusion

On the International Day of Persons with Disabilities, December 3, we once again affirm what defines us as an organization: the employment integration of individuals with disabilities, striving for their full integration in terms of employment, social participation, salary, and emotional well-being.
Assessment and approval of clinical trials in CTIS: Theoretical versus actual timelines

Managing clinical trials on the new European clinical trials platform, CTIS, involves taking into account not only the theoretical timelines foreseen in EU CTR 536/2014, but also the actual time it takes for Member States to assess and approve (if applicable) clinical trials submitted via this route.
The CDISC Standards: The Success Formula for Clinical Data
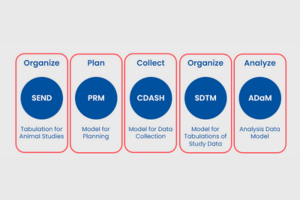
In the world of clinical research, data exchange and analysis play a key role in the development of safe and effective treatments. The Clinical Data Interchange Standards Consortium (CDISC) have proven to be the formula for success in achieving effective harmonization and standardization in clinical data exchange. In this article, we will explore how CDISC standards have revolutionized the way data is presented, analyzed and shared in medical research, improving the quality of clinical trials and accelerating progress towards new therapeutic advances.