
Our Blog
Are we making the most of the new European clinical trials platform, CTIS?
We analyze the usage data of the CTIS in its first three months of operation and we present you the new report that the EMA will publish periodically with the main KPIs. It comprehensively monitors the European clinical trial ecosystem.
By Lidya Domínguez
Regulatory Unit Head en Sermes CRO
We analyze the usage data of the CTIS in its first three months of operation and we present you the new report that the EMA will publish periodically with the main KPIs. It comprehensively monitors the European clinical trial ecosystem.
By Lidya Domínguez
Regulatory Unit Head en Sermes CRO
It has been a short time since the new European clinical trials platform, CTIS (Clinical Trial Information System), began operating. According to the data published by the EMA, from the launch of the CTIS (31st January) to 30th April, 56 clinical trials were submitted for assessment by Member States through CTIS, of which four were authorized.
The arrival of CTIS represents an absolute paradigm shift for clinical research operating in Europe, since it has consequences for both sponsors and regulatory agencies, as well as citizens and patients.
- The sponsors, once the transition period of three years has been exhausted, will have to carry out all the documentation procedures related to their clinical trials through CTIS.
- The regulatory agencies (which are the equivalent of what in the CTIS and CTR environment are referred to as Member States) will have to carry out their assessment through the new platform.
- The public will have access to more information about clinical trials since it will be available on the platform to the general public.
Transparency for the public and greater trust between the regulatory agencies
The submission of information on clinical trials is, therefore, one of the great novelties of this new scenario for research in Europe. The data is visible to patients, healthcare professionals and the general public through public search within CTIS. As published by the EMA “the public availability of an unprecedented amount of data on individual trials through CTIS empowers patients to find recruiting trials in their country and ensures the highest levels of transparency for clinical trials in Europe.”
A new framework of close collaboration is also opened – and a need for trust – between the regulatory agencies of the different Member States. This happens in the case of multinational trials, in which the sponsor must “choose” the notifying Member State, meaning that, the regulatory agency of that state will be solely responsible for assessing it. The agencies of the rest of the countries must abide by the result of this assessment.

KPIs for monitoring the European clinical trial ecosystem
Following the great effort made by the EMA and the Member States to implement this new clinical trial documentation management system, it is equally important to carry out an adequate monitoring of the progress achieved. Therefore, since last May, the EMA publishes on a monthly basis a report with the main KPIs of this transition. They can be consulted at this link.
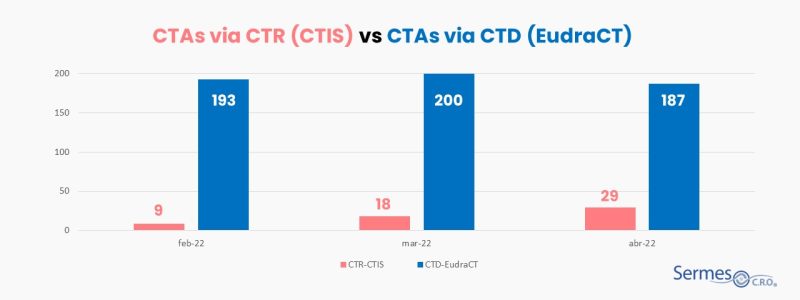
KPIs measure the number of clinical trial applications that have been submitted through CTR 536/2014 (meaning, through CTIS) and those that have been submitted under the Directive (meaning through EudraCT), since 31st January 2022. In addition, the report also offers data such as the number of authorized trials that are mono-national and multinational, commercial and non-commercial, and the number of trials per phase and therapeutic area.
The data published in the EMA’s first KPI report show that CTIS is starting to be used, but, logically, the figures for applications for authorization of clinical trials are definitely higher if we look at the figures from the old Directive. If 56 applications have been submitted by the CTIS in the three months studied (February/March/April 2022), a total of 580 have been submitted through EudraCT. That is, less than 10% of the total requests have been made through the new platform.
It is logical that the industry takes its time to adapt to the new situation, as there are many documents, procedures and internal workflows that must be modified. In fact, the standard provides for a transition period of 3 years, starting from 31st January 2022. However, it is also true that those who first start using the CTIS will be the ones who will be most benefited from the time and cost savings that this centralization of the management of all the documentation associated with the tests entail. In Sermes CRO we are already working with the tool. In fact, on 24th March we already presented our first trial by CTIS. The first of many!











