
Our Blog
From data recording to changing people's lives: 25 years of Sermes CRO
2022 is a big year for Sermes CRO. We are celebrating our 25th anniversary!! Antonio Berlanga, our CEO, was one of the two people that founded the company back in 1997. We now have more than 250 employees and have managed mora than 1500 clinical trials during the last five years. Learn more about our history and our future reading the full article!
By Antonio Berlanga
CEO at Sermes CRO
2022 is a big year for Sermes CRO. We are celebrating our 25th anniversary!! Antonio Berlanga, our CEO, was one of the two people that founded the company back in 1997. We now have more than 250 employees and have managed mora than 1500 clinical trials during the last five years. Learn more about our history and our future reading the full article!
By Antonio Berlanga
CEO at Sermes CRO

When we founded the company in 1997 to provide data recording services, we still did not know that, after two years, we were going to create our Clinical Trials Start-Up Unit. Since then, 25 years have passed, time enough to become a reference CRO for some of the most important pharmaceutical companies in the industry. Being part of the clinical research sector gives us reasons to work blissfully believing that we are contributing to improving people’s quality of life.
The goal of improving people’s lives is always present in every action we take: from the simple filing of a document to complex advising on, for example, the best strategy for a clinical trial with genetically modified organisms. That is how we have managed more than 1500 clinical trials in the last five years.
Obviously, we do not take care of the actual clinical part, although there are great experts among our employees… But we do take care of helping sponsors to carry out their research efficiently, with quality, within the framework of good clinical practice, complying with the requirements of regulatory agencies, observing national and European regulations… and with an eye on costs. Quite a challenge as well as a great satisfaction for the whole team!
Clinical research services
As a CRO (Contract Research Organization), we provide services to companies that conduct clinical research, such as pharmaceuticals, biotech and independent research. As known, being clinically successful is only part of what a promoter needs to be able to develop and market any new treatment or drug. In addition to a satisfactory clinical result, there is a whole part of documentation management and submission to regulatory agencies that is equally critical for trials to be approved. And that is exactly what we do.
To be able to achieve these, we rely on the best team: a group of highly qualified, experienced and motivated people… and with a human quality that makes us come to work (or do it from home) always with the best of moods. We are lucky to count on the professionalism of biotechnologists, doctors, pharmacists, biologists, chemists, psychologists, biochemists, engineers, veterinarians and nutritionists… They are all part of our staff and make us feel proud, every day, with their contribution, their experience, and their ability to produce ideas to improve clinical research processes.
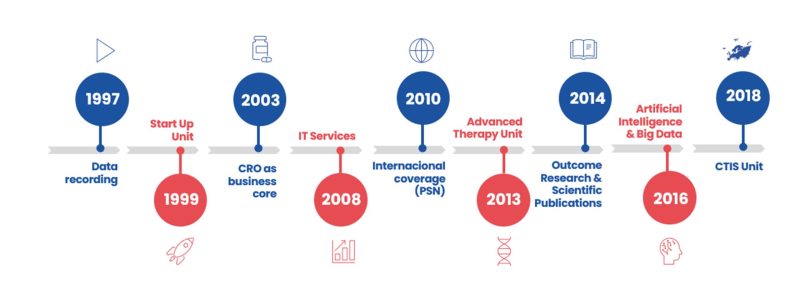
Our milestones
- 1997: Sermes was founded as a data entry service provider.
- 1999: Set up of Start Up Unit.
- 2003: CRO is already our business core. Monitoring, Pharmacovigilance, Biometry, Quality Control and Documentation activities.
- 2008: IT Services and Technology driven solutions.
- 2010: PSN Research was founded in order to deliver international coverage.
- 2013: Advanced Therapy Unit.
- 2014: Outcome Research and Scientific Publications activities.
- 2016: Artificial Intelligence and Big Data Unit.
- 2021: EU Clinical Trial Regulation / CTIS related services.
Experience as CRO + Artificial Intelligence: the perfect couple for clinical research
Innovative is a good adjective to describe how Sermes CRO has been since the beginning. We do not know how to work without always setting foot in the future. That is why it has been already 12 years since we started offering technological services to our clients and more than 5 since we launched our Big Data and Artificial Intelligence Unit.
We are very clear about the value we bring: being able to combine our experience as CRO (knowing very closely what are the most pressing needs of clinical trial promoters, as well as their stoppers) and our knowledge in Artificial Intelligence. This helps us to create solutions that a few years ago seemed like science fiction. Today we know that this type of AI solutions can help speed up clinical research and facilitate access to treatments in record time. That is our everyday motivation and the reason why we can celebrate our 25 anniversary with the hope of continuing to do our bit to make this world a better place to live.











