
Our Blog
How to register with OMS to be able to work with CTIS?
To be able to carry out a clinical trial in CTIS, it is previously necessary to be registered with OMS. In this article we explain the OMS registration process for sponsors and sites participating in clinical trials
By Blanca Oñoro
Clinical Documentation and Advance Therapy Technician at Sermes CRO
To be able to carry out a clinical trial in CTIS, it is previously necessary to be registered with OMS. In this article we explain the OMS registration process for sponsors and sites participating in clinical trials
By Blanca Oñoro
Clinical Documentation and Advance Therapy Technician at Sermes CRO

With the implementation of CTIS (the new European clinical trials portal) as the single-entry point for the management of clinical trials documentation in Europe, there are many new features that must be taken into account by all of the sponsors, sites, and CROs which are dedicated to clinical research. One of these new features is the mandatory prior registration with OMS in order to operate within CTIS.
What is OMS (Organisation Management Services)?
To be able to carry out a clinical trial under Regulation (EU) 536/2014, the trial sponsors (along with the service providers to which any of their functions were delegated) and the sites participating in the study must be registered with OMS (Organisation Management Services).
OMS is a portal that contains a dictionary with validated information on numerous organizations. It includes not only the names of the different organizations, but also their location details and contact information (such as e-mail address and telephone number). Each organization is assigned a number, called the ORG-ID.
This platform allows you to view, search, export organization data, request the registration of a new organization or update the data of an existing organization.
In this way, OMS favors continuous and centralized access to validated information from different organizations such as commercialization authorization holders, sponsors, regulatory authorities, trial sites and manufacturers.
What is the relation between CTIS and OMS?
One of the main purposes of the Clinical Trials Regulation consists in harmonizing the submission, assessment and review of the processes related to clinical trials in the European Union (EU) through the new portal, called CTIS (Clinical Trials Information System).
To carry out the application for the authorization of a trial, the data of the various organizations involved must be included in CTIS. CTIS collects the data directly through OMS. Therefore, the sponsors of the trial, along with the service providers (such as Contract Research Organizations – CROs, or other facilities participating in the conduct of the study) must have successfully registered with OMS to be able to apply for the authorization of a trial. Additionally, the sites which participate in the clinical trial must be included in CTIS too, and their information is also obtained from OMS, therefore these sites are also considered as organizations that need to be registered in this portal.
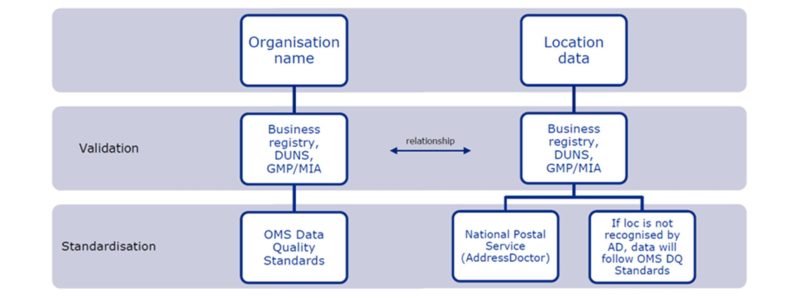
What is the process to register an organization with OMS?
If an organization is not yet registered with OMS when starting to use CTIS, the registration must be carried out. There are two ways to carry out the registration of an organization:
- Directly through the OMS portal.
- While working in CTIS.
Regardless of the route used to carry out the registration, the necessary documentation which supports the process must always be provided (this documentation will depend on the type of organization to be registered, and whether or not the organization is registered in a national business registry), and the application will be validated within a maximum of 10 business days:
- For organizations located in European Economic Area (EEA) countries, the name of the organization will be validated against the national business registry (where available) and the data (e.g., the name of the organization) will be standardized according to OMS data quality standards.
- For organizations located in non-EEA countries, the name of the organization will be validated with the DUNS website (when available) and the data, as above, will also be standardized according to OMS data quality standards.
- The organization’s location data will be validated and standardized by the OMS address verification tool (AddressDoctor).
In case the submitted application is incorrect or not supported by proper documentation, the validation in OMS will fail and the organization’s data will not be registered in OMS.
What is new in the registration process of organizations in OMS?
As of the 3rd of November 2022, OMS allows any sponsor that is not registered in a national business registry (e.g., in the case of organizations based in Spain that are not registered in the National Business Census) to use a specific application to register with OMS and allow their information to be included in CTIS. To do so, the application for registration must be made including the specific registration letter, called CT registration Headed letter (whose template is available on the OMS web page), with all the necessary information:
- The name of the organization and its address details.
- Public information regarding the organization, e.g., information available at fda.gov or local clinic/physician registries.
- The organization’s contact details (e.g., website/link/public e-mail).
- Information regarding the clinical trial.
In this way, all sponsors that are not registered in a national business registry will be able to register with OMS without the need for a name validation process. OMS will maintain registrations for a sponsor active as long as it can be verified that the sponsor’s information is currently being used in a CTIS trial application. This verification will be performed during the periodic OMS revalidation, with the goal of ensuring that organizations that have remained unchanged for more than 2 years are still valid. Please note that any sponsor registered via the CT registration Headed letter will be deactivated if not used in CTIS as a sponsor or if contact cannot be established after 3 consecutive attempts via the e-mail provided upon registration.
In addition, as of the 3rd of November 2022 OMS has also implemented a temporary process to support the registration of clinical trial sites that are not registered in a national business registry, following the same process as described above for sponsors, whereby the name of the organization does not need to be validated. This will be possible until a new functionality is implemented in CTIS, scheduled for the 6th of December. This functionality will allow the registration of clinical trial sites directly in CTIS.
Once the CTIS change is implemented, the OMS team will deactivate all clinical trial sites registered through the CT registration Headed letter and these organizations will not be subject to any further periodic revalidation checks. Only sites that can be validated by national business registries will remain active in the OMS dictionary. In addition, only new centers that can be validated by national business registries will be registered and all site registrations that are applied for via the CT registration Headed letter will be rejected.











